How to bio-engineer a football
Wed 29 Aug 2012Historically, footballs have been improvised from a great many materials – but growing a football from living pig bladder cells?
It’s a long story but, basically, I wanted to develop a new kind of football which would retain a link with the game’s raw heritage, but, at the same time, embrace and explore the potential for using tools of contemporary biotechnology.
Several stages are involved in the production of my bio-engineered footballs: from organic origins through digital manufacturing and controlled growth under laboratory conditions. Below are some stages towards this new kind of consumer object.
1. Harvesting stem cells from pig bladder

A crucial first step is in obtaining viable, smooth muscle, stem cells which can then be ‘cultured’ – grown – in the laboratory in Liverpool. Although a very wide variety of cells are available commercially (and stored globally for use in research and experimentation) it became important in this work to explore the process of production from beginning to end: when it comes to biotechnology, the beginning of the process is with the animal. I made several trips to abattoirs in South Yorkshire and, later, near Warrington to get animal bladders immediately after slaughter. This is the crucial raw material.
In science, the term used for a ‘recipe’ is protocol – protocols explain how to do a certain procedure correctly. I worked with an existing protocol for obtaining viable smooth muscle stem cells from the bladders of rats and mice and, with advice from colleagues at the lab, I adapted this to suit my locally sourced pig bladders.
The procedure involves precisely chopping up small amounts of the bladder muscle tissue and then using an enzyme to break down the structure which holds the cells in place. Debris is filtered away and cells are then placed in a culture medium – a kind of food for cells. At every stage, there is a high possibility that the tissue will become contaminated (and this happened twice before I finally succeeded in securing the cells used for the production of the work.)
2. Culturing pig bladder smooth muscle stem cells in laboratory
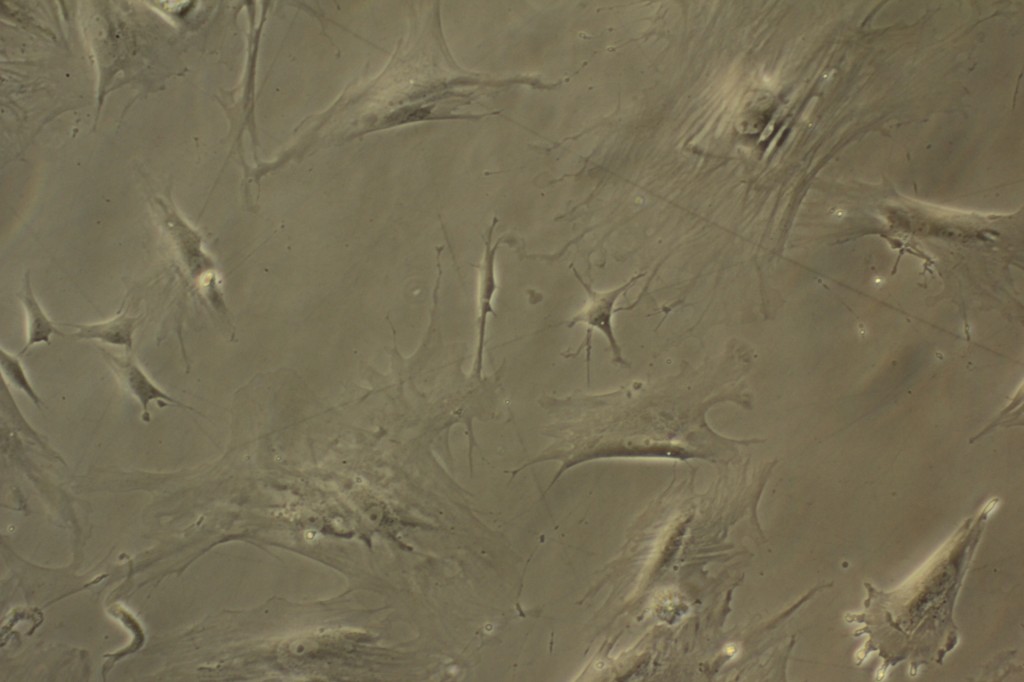
When growing living animal cells in the laboratory an analogy might be drawn from gardening. The cells need a controlled environment (37 degrees Celsius and 5% carbon dioxide in air), food (fetal calf serum) and water (supplied via airborne humidity).
In order to produce the time-lapse imagery of cell growth seen in the Pigs Bladder Football exhibition, I had to build an incubation environment where I could control each of these factors and also contain the microscope and camera.
I grew different strains of cells for the whole year – from my first day in the lab in August 2011 up until the day before the exhibition! These included L929 (an ‘immortalised’ mouse muscle cell strain which I recovered from 8 years of being abandoned in the cryogenic freezer), bone marrow stem cells obtained from a pig knee joint bought at a butchers, and the pig bladder cells – which were the most challenging to harvest and maintain.
The cells typically need fresh medium every 5-10 days (during which time they have doubled their numbers many times and filled the tissue culture flasks). If you’re not careful, this kind of geometric growth can result in a ‘sorcerer’s apprentice’ situation, with cell numbers getting out of hand!
3. Design and generation of scaffold forms:
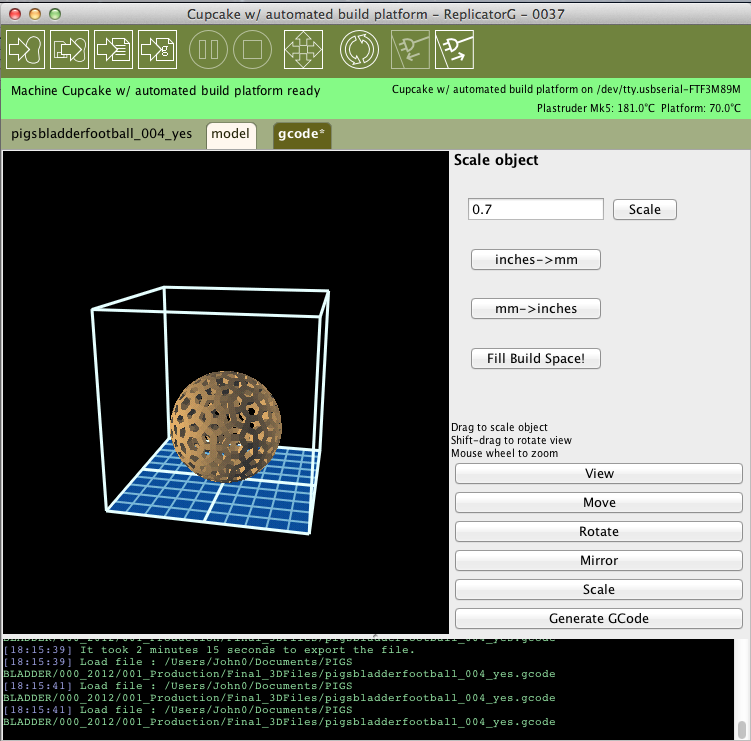
In tissue engineering, mesh scaffolds are used in order to encourage the growth of animal tissues towards a desired form for re-integration into the body. In this case, I was basically trying to create a sphere, but I wanted to incorporate the hexagons and pentagons of a traditional football and also suggest the organic branching of vascular systems. I worked with a wide range of digital design tools to generate three-dimensional shapes incorporating a mesh onto which the cells might grow.
4. 3D printing of biodegradable football scaffold
The video above (shot on my phone) shows the 3D printer (a MakerBot named Cupcake) in operation at DoES Liverpool – a fantastic workshop and makerspace in Liverpool city centre. DoES has been really supportive in providing expertise and access to equipment for developing various prototype 3D scaffolds.
I have also been working with very high specification 3D sintering machine (at Liverpool University) but, at this phase of my project, I’m increasingly interested in the possibilities for production (in fields of bio-science) outside of traditional institutional structures. It’s my opinion that, the more these kinds of techniques can be accessed, shared and explored by non-specialists (like myself) in DIY scenarios, the more interesting questions will arise regarding the practical and ethical uses for tissue engineered products.
5. Seeding of 3D forms with stem-cells and ‘growing’ footballs in bioreactor
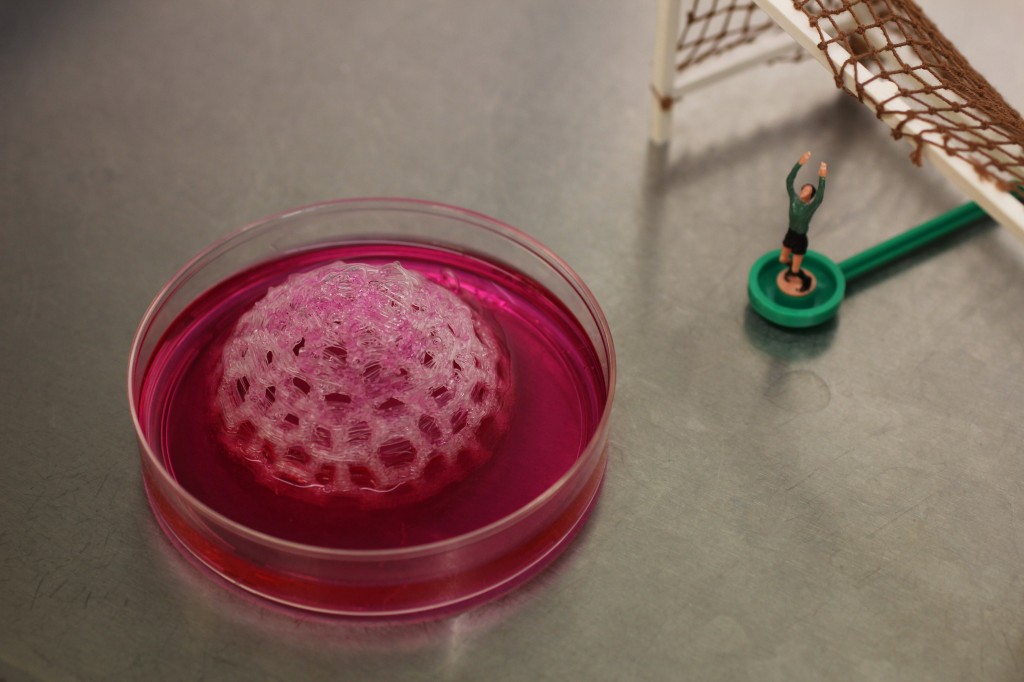
The real challenge in all of this bio-science work has been attempting to control and limit the many factors which can mean the difference between success and failure.
In traditional sculpture, a craftsperson works with a very stable resistant material (stone or metal) whereas, to work with biotechnology sculpturally, a large amount of direct control has to be relinquished. Instead, the artist designs an experiment, and it is from there that a form can emerge.
I have made numerous tests to determine what might encourage the pig bladder cells to adhere to the PLA scaffold material and grow within the improvised bio-reactors that I am using – but what we will have to show within the premiere of the exhibition, only time will tell. Fingers crossed!
John O’Shea, August 2012
A key part of the AND 2012 Exhibitions programme, Pigs Bladder Football launches Wed 29 Aug, 6pm at CUBE, Manchester and is open free until 07 Sep. A free artist talk ’21st Century Football’ will take place Sat 01 Sep, 2pm at the National Football Museum.
For further details visit: www.andfestival.org.uk/events/pigs-bladder
Recent Journals
- A Gig at Sunrise: Reflecting on W Brzask at Ephemera Festival
- Announcing our THREE FIELDS artists
- New Rhythms
- Introducing Commons // Keiken and Jazmin Morris
- Introducing our Creative Associates programme
- Reflections on the Associate Board Member Programme
- The Future of Arts Governance
- Rendering our virtual, net and digital discourses
- Announcing a new partnership between AND and the School of Digital Arts
Other Journals
-
2025
-
2024
-
2023
-
2022
-
2021
-
2020
-
2019
-
2018
-
2017
-
2016
-
2015
-
2014
-
2013
-
2012
-
2011



